![]()
The Abiotic Hydrolysis Reaction Library has been developed as a component of the Chemical Transformation Simulator (CTS), a web-based software tool under development in EPA’s Office of Research and Development. The library is implemented in CTS to predict the likely hydrolytic transformation products in the environment for an organic chemical of interest.
Version 1.8 of the Abiotic Hydrolysis Reaction Library contains 24 reaction schemes:
· Halogenated Aliphatics: Nucleophilic Substitution
o Scheme A: C-X with no other adjacent halogens
o Scheme B: C-X with vicinal halogen atoms
o Scheme C: C-X with geminal halogen atoms
· Halogenated Aliphatics: Elimination
· Organophosphorus Ester Hydrolysis 1 (Base-Catalyzed)
· Organophosphorus Ester Hydrolysis 2 (Neutral or Acid-Catalyzed)
· Carboxylic Acid Ester Hydrolysis
Ranking of Hydrolysis Reaction Schemes
The reaction schemes are written as generic reaction equations defining how a particular structural fragment will be modified by the transformation reaction. These schemes are not balanced reactions showing all reactants and products (e.g., H2O, OH- and/or H+ are not shown as reactants in the hydrolysis schemes). Additionally, the structural fragments in the reaction schemes are written with a minimal amount of specificity. For example, the inclusion of hydrogen atoms in the scheme implies that there is a requirement for a hydrogen atom to present be in the specified position for the reactions to proceed; otherwise, it is assumed that, for simplicity, hydrogen atoms are not explicitly included.
The schemes are encoded using the notation and structural query features (L, ~L, L1-X, etc) from ChemAxon’s Marvin tools. Definitions of some common symbols used in the reaction schemes are provided below:
· L[a1;a2;…] is a list of possible atoms (a1, a2, …) that can occupy the position within the fragment
· ~L![a1;a2;…] is a list of atoms (a1, a2, …) that cannot occupy the position within the fragment
· A is any atom except hydrogen
· AH is any atom including hydrogen
· X is any halogen
· (A) is used to indicate an aliphatic carbon atom
· (a) is used to indicate an aromatic carbon atom
· (L1-N) is used to indicate a string of atoms (acyclic or cyclic) of length N
· (X#) is used to indicate the # of connections (= substituents including hydrogen) attached to the atom
· (s#) indicates the substituent count on the atom
· (s*) indicates substituent count is as drawn for the atom
Examples are provided for each reaction scheme in the library. As is the case for the reaction schemes themselves, the example reactions do not show all of the reactants and products involved in the hydrolysis reaction. The example chemical is shown as the only reactant, and the products are the major hydrolysis products reported in the study. These example transformations from the peer-reviewed literature and government regulatory reports were used to test the reaction schemes in the library.
The schemes within the hydrolysis library are ranked on a scale of one to seven according to their relative rate of transformation, with a higher rank indicating a faster hydrolysis rate. A database of measured rate constants or half-lives was compiled from a survey of peer-reviewed scientific literature and reports by government regulatory agencies to assign these ranks to each hydrolysis reaction scheme. Separate rank assignments are established for pH 5, 7 and 9 to represent standard conditions in hydrolysis studies required for registration of pesticides in OECD member countries.
Halogenated Aliphatics: Nucleophilic Substitution
The replacement of a halogen atom with an OH group through the nucleophilic substitution mechanism is represented by three schemes within the Abiotic Hydrolysis Reaction Library to capture the effects of molecular structure on reactivity in the immediate vicinity of the carbon-halogen bond. The removal of a halogen from a carbon atom with no other halogens in vicinal and geminal positions, represented by Scheme A, occurs at a faster rate than removal of halogen with other halogens in the vicinity of the reaction site. The removal of halogen atoms with other halogens in vicinal and geminal positions are represented by Schemes B and Scheme C, respectively. Observed half-lives for the hydrolytic removal of fluoride atoms under environmentally relevant conditions generally exceed 20 years and are often in the range of hundreds of years; therefore, no scheme is included for the removal of fluoride atoms.
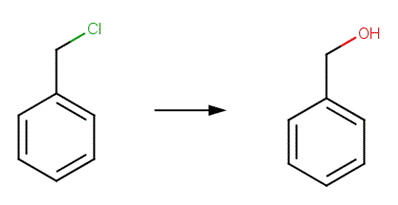
SCHEME A:
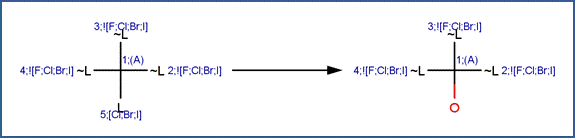
To differentiate Scheme A from Scheme B (for vicinal dehalogenation), this scheme includes an exclude rule specifying that reactant atom #1 is not part of the pattern X-C-C-X, where X represents a halogen.
EXAMPLES FOR SCHEME A:
· methyl bromide (EFSA, 2006; U.S. EPA, 1992)

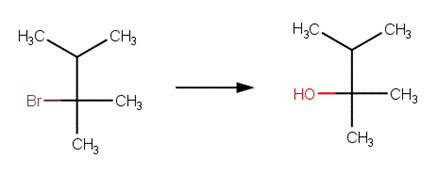
· 2‐bromo‐2,3‐dimethylbutane (McMurry, 2011, p. 372)
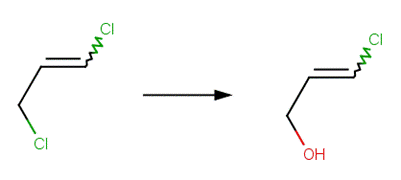
· 1,3-dichloropropene (EFSA, 2004; Guo et al, 2004)
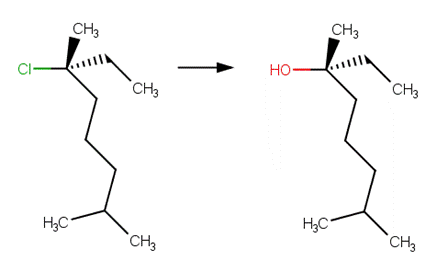
· (6R)‐6‐chloro‐2,6‐dimethyloctane (McMurry, 2011, p. 388)
· benzyl chloride (U.S. EPA, 1992)
SCHEME B:
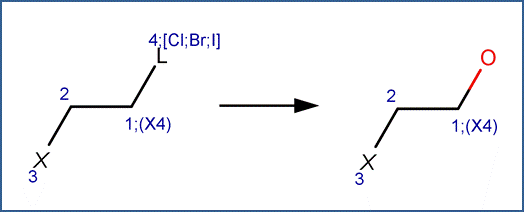
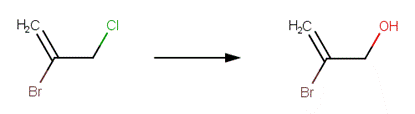
This scheme includes an exclude rule to distinguish the scheme from Scheme C for dehalogenation at a reaction site with more than one halogen attached to the same carbon. The exclude rule specifies that atom #1 can only be attached to one halogen.
This scheme also includes a selectivity rule to indicate the order of removal of halogens for molecules with different halogen substituents. The order of removal of the leaving halogen (labeled reactant atom 4 in the scheme) is inverse to its atomic number, i.e., I>Br>Cl. This is due to the fact that the carbon-halogen bond strength is greatest for the most electrophilic halogen. (Larson and Weber, 1994)
EXAMPLES FOR SCHEME B:
· 2-bromo-3-chloropropene (Burlinson et al, 1982)
SCHEME C:
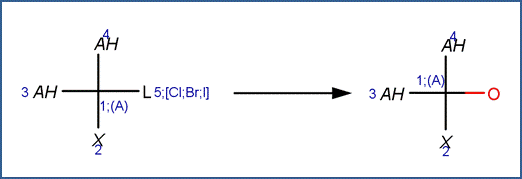
This scheme includes a selectivity rule to indicate the order of removal of halogens for molecules with different halogen substituents. The order of removal of the leaving halogen (labeled reactant atom 5 in the scheme) is inverse to its atomic number, i.e., I>Br>Cl. This is due to the fact that the carbon-halogen bond strength is greatest for the most electrophilic halogen. (Larson and Weber, 1994)
EXAMPLES FOR SCHEME C:
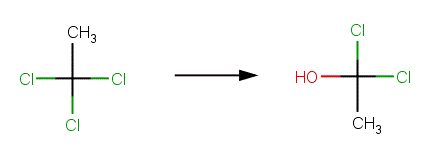
· 2,2-Dichloropropane (Ellington et al, 1988) Reported products are 2-chloropropene (due to elimination) and acetone. The example shown below, with formation of 2-chloro-2-propanol, is the first step in the formation of acetone. 2-Chloro-2-propanol is hydrolyzed according Nucleophilic Substitution Scheme A to 2,2-propanediol, which is a geminal diol that rapidly undergoes dehydration to form acetone.
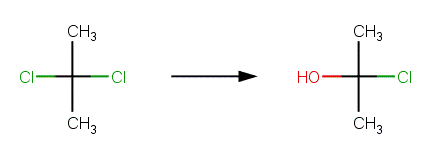
· 1,1,1- trichloroethane (Cline et al, 1986; Cline and Delfino, 1989; Dilling etal, 1975) Reported products are acetic acid and a minor amount of vinylidene (due to elimination). The example shown below, with formation of 1,1‐dichloroethanol, is the first step in the formation of acetic acid. 1,1‐dichloroethanol is hydrolyzed according Nucleophilic Substitution Scheme A to 1‐chloroethane‐1,1‐diol, which is a geminal diol that rapidly undergoes dehydration to form acetyl chloride. Acetyl chloride is rapidly hydrolyzed to acetic acid according to the Acid Halide Scheme.
REFERENCES:
Burlinson, N.E., L.A. Lee and D.H. Rosenblatt. 1982. Kinetics and products of hydrolysis of 1,2-dibromo-3-chloropropane. Environmental Science and Technology. 16(9): 627-632.
Cline, P.V., J.J. Delfino and W.J. Cooper. 1989. Transformation kinetics of 1,1,1- trichloroethane to the stable product 1,1- dichloroethene. In R.A. Larsen, ed. Biohazards of Drinking Water Treatment. Chelsea, MI: Lewis Publishers, Inc. pp. 47-56.
Cline, P.V., J.J. Delfino and W.J. Cooper. 1986. Hydrolysis of 1,1,1- trichloroethane; formation of 1,1- dichloroethene. Proceedings of NWWA/API Conference on Petroleum Hydrocarbons and Organic Chemicals in Ground Water: Prevention, Detection and Restoration (Dublin, OH), 239-247.
Dilling, W.L., N.B. Tefertiller and G.J. Kallos. 1975. Evaporation rates and reactivities of methylene chloride, chloroform, 1,1,1-trichloroethane, trichloroethylene, tetrachloroethylene, and other chlorinated compounds in dilute aqueous solutions. Environmental Science and Technology. 9(9): 833-838.
Ellington, J.J., F.E. Stancil, W.D. Payne and C.D. Trusty. 1988. Measurement of hydrolysis rate constants for evaluation of hazardous waste land disposal: Volume 3. Data on 70 chemicals. U.S. Environmental Protection Agency. EPA/600/3-88/028.
EFSA (European Food Safety Authority), 2004. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Spain for the existing active substance 1,3-Dichloropropene of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 5, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2006. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State The United Kingdom for the existing active substance Methyl Bromide of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
Guo, M., S.K. Papiernik, W. Zhang and S.R. Yates. 2004. Effects of environmental factors of 1,3-dichloropropene hydrolysis in water and soil. Journal of Environmental Quality. 33(2): 612-618.
Larson, R.A. and E.J. Weber. Reaction Mechanisms in Environmental Organic Chemistry. Boca Raton: CRC Press, Inc., 1994.
McMurry, J.E. 2011. Organic Chemistry, 8th ed. Boston, MA: Cengage Learning.
U.S. EPA (United States Environmental Protection Agency). 1992. Environmental fate constants for organic chemicals under consideration for EPA’s Hazardous Waste Identification Rule. EPA/601/R-92/006.
Halogenated Aliphatics: Elimination
SCHEME:
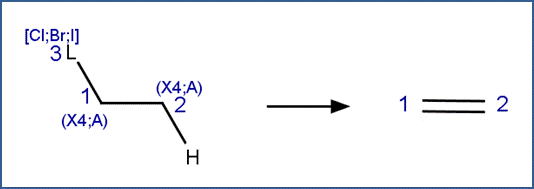
This scheme includes two selectivity rules:
1. The carbon atom with the hydrogen leaving group (labeled reactant atom 2 in the scheme) is the one that has the most steric hindrance. In effect, this is Zaitsev’s Rule, which states that “The alkene formed in greatest amount is the one that corresponds to removal of the hydrogen from the β-carbon having the fewest hydrogen substituents.” (Reusch, 2010)
2. The order of removal of halogens (labeled reactant atom 3 in the scheme) is inverse to their atomic number, i.e., I>Br>Cl. This is due to the fact that the carbon-halogen bond strength is greatest for the most electrophilic halogen. (Larson and Weber, 1994)
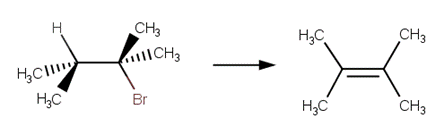
EXAMPLES:
· 2‐bromo‐2,3‐dimethylbutane (McMurry, 2011, p. 372; Reusch, 2010)
· 2‐bromobutane (McMurry, 2011, p. 397; Reusch, 2010)

· 2‐bromo‐2‐methylbutane (McMurry, 2011, p. 397)
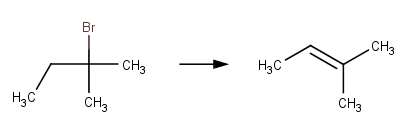
· 1‐chloro‐1‐methylcyclohexane (McMurry, 2011, p. 399)
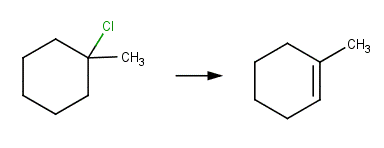
· DDD (dichlorodiphenyldichloroethane) (U.S. EPA, 1992)
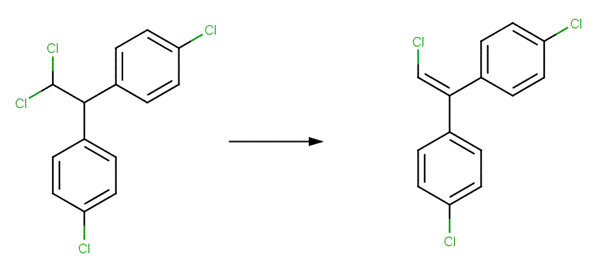
· 1,2‐dichloroethane (U.S. EPA, 1992; Miyamoto and Urano, 1996)
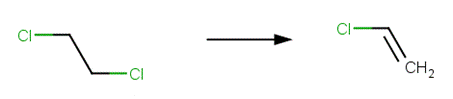
· 1,1,2,2-tetrachloroethane (Cooper et al, 1987)
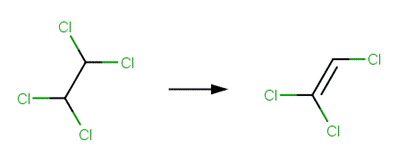
· 1,1,1-trichloroethane (Cline and Delfino, 1989; Gerkens and Franklin, 1989; Miyamoto and Urano, 1996)
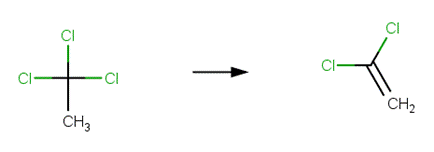
· 1,2-dibromo-3-chloropropane (Burlinson et al, 1982)
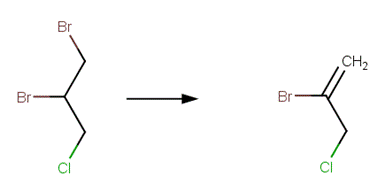
REFERENCES:
Burlinson, N.E., L.A. Lee and D.H. Rosenblatt. 1982. Kinetics and products of hydrolysis of 1,2-dibromo-3-chloropropane. Environmental Science and Technology. 16(9): 627-632.
Cline, P.V. and J.J. Delfino. 1989. Transformation kinetics of 1,1,1-trichloroethane to the stable product 1,1-dicheloroethene. In Larsen, R.A., editor, Biohazards of Drinking Water Treatment. Lewis Publishers, Inc., Chelsea, Michigan, pp. 47-56.
Cooper, W.J., M. Mehran, D.J. Riusech and J.A. Joens. 1987. Abiotic transformation of halogenated organics: 1. Elimination reaction of 1,1,2,2-tetrachloroethane and formation of 1,1,2-trichloroethene. Environmental Science and Technology. 21(11): 1112-1114.
Gerkens, R.R. and J.A. Franklin. 1989. The rate of degradation of 1,1,1-trichloroethane in water by hydrolysis and dehydrochlorination. Chemosphere. 19(12): 1929-1937.
Larson, R.A. and E.J. Weber. Reaction Mechanisms in Environmental Organic Chemistry. Boca Raton: CRC Press, Inc., 1994.
McMurry, J.E. 2011. Organic Chemistry, 8th ed. Boston, MA: Cengage Learning.
Miyamoto, K. and K. Urano. 1996. Reaction rates and intermediates and chlorinated organic compounds in water and soil. Chemosphere. 32(12): 2399-2408.
Reusch, W.H. 2010. Virtual Textbook of Organic Chemistry. http://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/intro1.htm.
U.S. EPA (United States Environmental Protection Agency). 1992. Environmental fate constants for organic chemicals under consideration for EPA’s Hazardous Waste Identification Rule. EPA/601/R-92/006.
SCHEME:
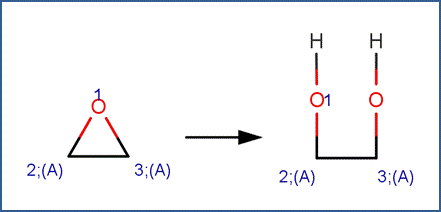
EXAMPLES:
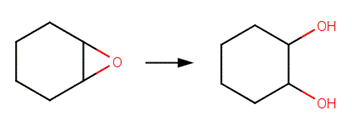
· 1,2-Epoxycyclohexane (McMurry, 2011)
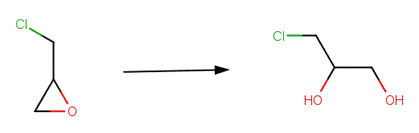
· Epichlorohydrin (Gaca et al, 2011)
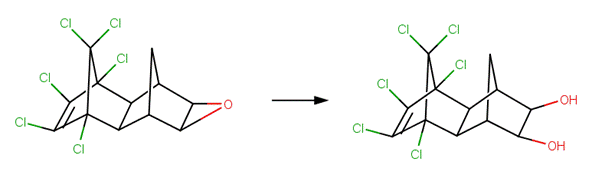
· Endrin (Larson and Weber, 1994; U.S. EPA, 1992)
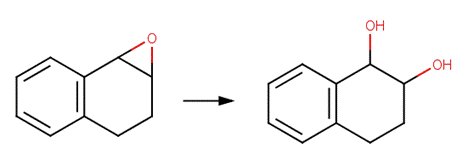
· 1,2-Epoxy-1,2,3,4-tetrahydronaphthalene (Becker et al, 1979)
REFERENCES:
Becker, A.R., J.M. Janusz and T.C. Bruice. 1979. Solution chemistry of the syn- and anti-tetrahydrodiol epoxides, the syn- and anti-tetrahydrodimethoxy epoxides, and the 1,2- and 1,4-tetrahydro epoxides of naphthalene. Journal of the American Chemical Society. 101(19): 5679-5687.
Gaca, J., G. Wejnerowska and P. Cysewski. 2011. Mechanism of the acidic hydrolysis of epichlorohydrin. Journal of Physical Organic Chemistry. 24: 1045-1050.
Larson, R.A. and E.J. Weber. Reaction Mechanisms in Environmental Organic Chemistry. Boca Raton: CRC Press, Inc., 1994.
McMurry, J.E. 2011. Organic Chemistry, 8th ed. Boston, MA: Cengage Learning.
U.S. EPA (United States Environmental Protection Agency). 1992. Environmental fate constants for organic chemicals under consideration for EPA’s Hazardous Waste Identification Rule. EPA/601/R-92/006.
Organophosphorus Ester Hydrolysis 1 (Base-Catalyzed)
SCHEME:
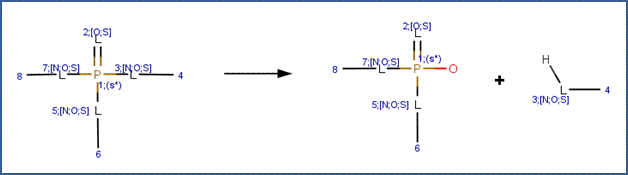
This scheme covers the base-catalyzed hydrolysis of all organophosphorus triesters, including phosphates, phosphorothiolates, phosphorothioates, phosphorodithioates and N derivatives, such as phosphorodiamidates. This scheme includes a selectivity rule to identify the most likely leaving group. Base-catalyzed cleavage favors P-L cleavage (where L is O, S, or N) at the L group that is attached to the most electron-withdrawing group (Larson and Weber, 1994). The selectivity rule specifies that the leaving group (labeled atom 3) is the attached the carbon atom (labeled atom 4) with the highest electrophilicity.
EXAMPLES:
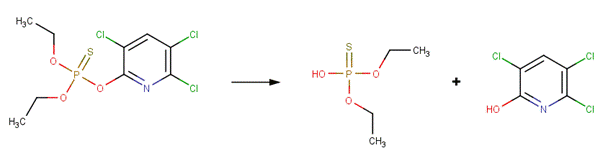
· Chlorpyrifos (Macalady and Wolfe, 1983)
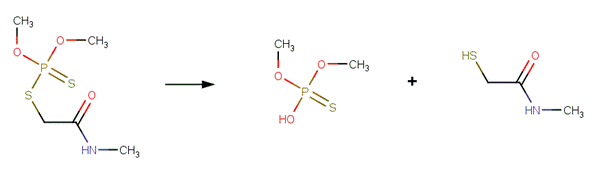
· Dimethoate (EFSA, 2005a)
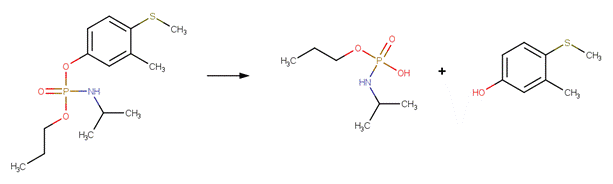
· Fenamiphos (EFSA, 2005b)
· Fenitrothion (EFSA, 2005c; Greenhalgh et al , 1980)
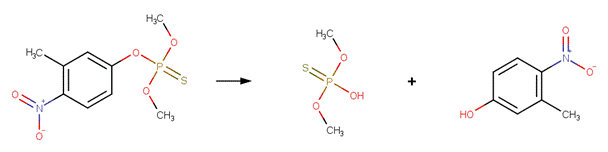
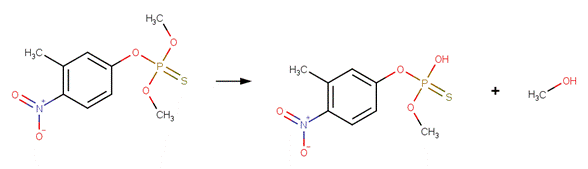
Organophosphorus Ester Hydrolysis 2 (Neutral and Acid-Catalyzed)
SCHEME:
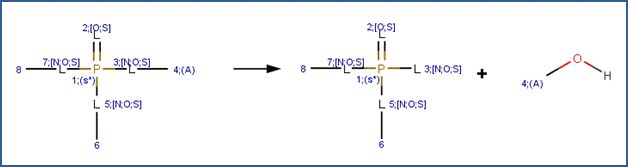
This scheme covers the neutral and acid-catalyzed hydrolysis of all organophosphorus triesters, including phosphates, phosphorothiolates, phosphorothioates, phosphorodithioates and N derivatives, such as phosphorodiamidates. This scheme includes a selectivity rule to identify the most likely leaving group. Neutral and acid-catalyzed cleavage favors L-C cleavage (where L is O, S, or N), but not at the L group that is attached to the most electron-withdrawing group (Larson and Weber, 1994). The selectivity rule specifies that the leaving group (labeled atom 4) is the carbon atom with the lowest electrophilicity.
EXAMPLES:
· Chlorpyrifos (Macalady and Wolfe, 1983)
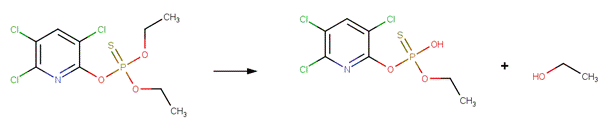
· Dimethoate (EFSA, 2005a)
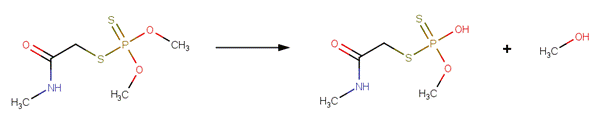
· Fenitrothion (EFSA, 2005c; Greenhalgh et al , 1980)
REFERENCES:
EFSA (European Food Safety Authority), 2005a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the United Kingdom for the existing active substance Dimethoate of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2005b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the Netherlands for the existing active substance Fenamiphos of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2005c. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the United Kingdom for the existing active substance Fenitrothion of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
Greenhalgh, R. K.L. Dhawan and P. Weinberger. 1980. Hydrolysis of fenitrothion in model and natural aquatic systems. Journal of Agricultural and Food Chemistry. 28(1): 102-105.
Larson, R.A. and E.J. Weber. Reaction Mechanisms in Environmental Organic Chemistry. Boca Raton: CRC Press, Inc., 1994.
Macalady, D.L. and N.L. Wolfe. 1983. New perspectives on the hydrolytic degradation of the organophosphorothioate insecticide chlorpyrifos. Journal of Agricultural and Food Chemistry. 31(6): 1139-1147.
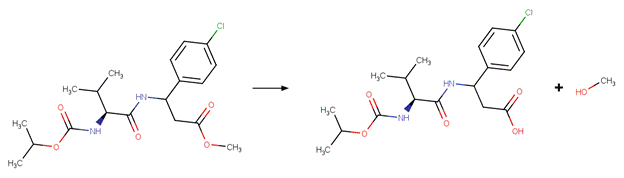
Carboxylic Acid Ester Hydrolysis
SCHEME:
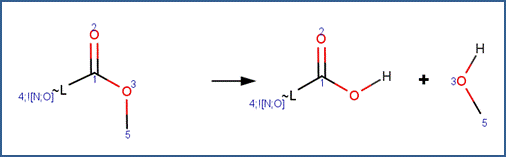
Two reactivity rules are included for this scheme. To distinguish this scheme from the Anhydride Hydrolysis scheme, the first reactivity rule specifies that atom 3 is not part of an anhydride structural fragment. To distinguish this scheme from the Lactone scheme, the second reactivity rule specifies that atom 3 is a chain atom.
EXAMPLES:
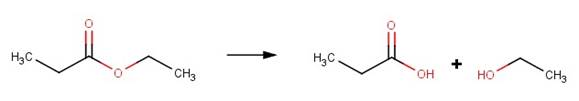
· Ethyl propanoate (McMurry, 2012)
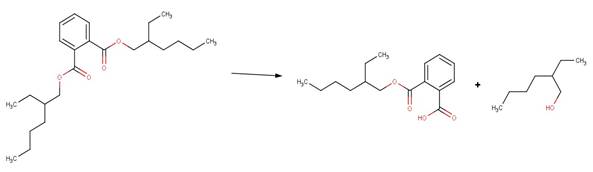
· Bis(2-ethylhexyl)phthalate (Larson and Weber, 1994)
· Fenpropathrin (Larson and Weber, 1994)
· Chlorobenzilate (Larson and Weber, 1994)
· 2,5-dichlorobenzoic acid methylester (EFSA, 2007a)
· Propaquizafop (EFSA, 2006)
· Trinexapac (EFSA, 2005)
· Diclofop-methyl (EFSA, 2007b)
· Fluroxypyr (EFSA, 2009)
· Kresoxim-methyl (EFSA, 2010)
· Valifenalate (EFSA, 2012)
REFERENCES:
Larson, R.A. and E.J. Weber. Reaction Mechanisms in Environmental Organic Chemistry. Boca Raton: CRC Press, Inc., 1994.
McMurry, J.E. 2011. Organic Chemistry, 8th ed. Boston, MA: Cengage Learning.
EFSA (European Food Safety Authority), 2005. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the Netherlands for the existing active substance Trinexapac of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.7, part 1. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2006. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Italy for the existing active substance Propaquizafop of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2007a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Germany for the existing active substance Dichlorobenzoic Acid Methylester of the third stage (part B) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 2, B.6. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2007b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State France for the existing active substance Diclofop-Methyl of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2009. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Ireland for the existing active substance Fluroxypyr upon submission in the framework of the renewal of the inclusion of a first group of active substances in Annex I to Council Directive 91/414/EEC in accordance with Commission Regulation (EC) No 737/2007, Volume 3, B7. http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2010. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Belgium for the existing active substance Kresoxim-Methyl of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2012. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Hungary for the existing active substance Valifenalate of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
SCHEME:
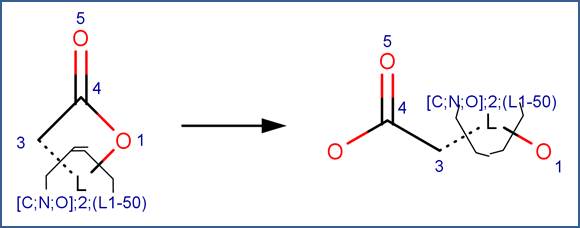
To distinguish this scheme from the Carboxylic Acid Ester scheme, the scheme includes a reactivity rule which specifies that atom 1 is a ring atom. Additionally, to distinguish this scheme from hydrolysis of cyclic anhydrides, the scheme includes a reactivity rule which specifies that atom 1 is not part of an anhydride structural fragment.
EXAMPLES:
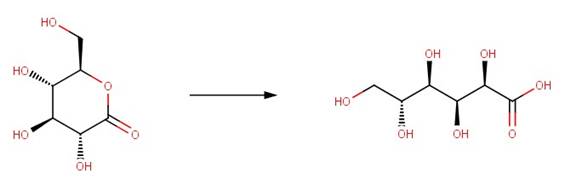
· Gluconolactone (Pocker and Green, 1973)
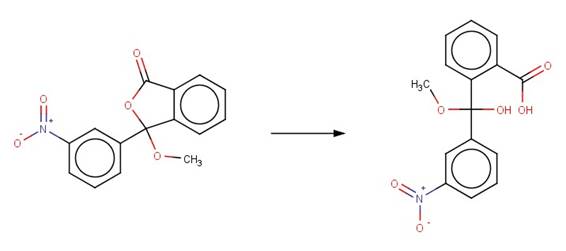
· 3-methoxy-3-(3-nitrophenyl)-2-benzofuran-1-one (Weeks and Whitney, 1981)
· (3Z)-3-{[(2,5-dimethylphenyl)sulfanyl]methylidene}-2-benzofuran-1-one (Bowden et al, 1998)
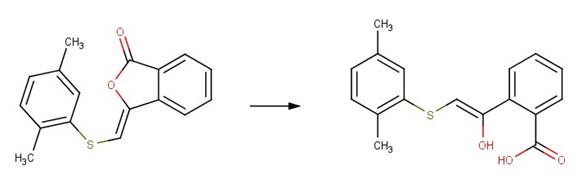
· (3E)-3-(4-methoxyphenoxymethylidene)-2-benzofuran-1-one (Bowden et al, 1998)
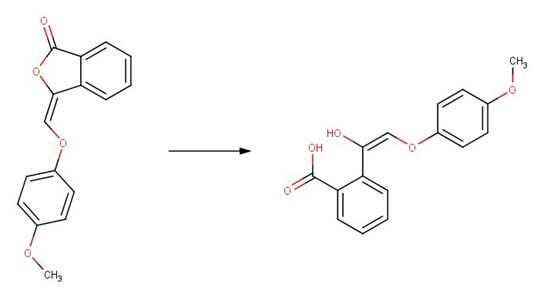
· Beta-butyrolactone (Olson and Voule, 1951)
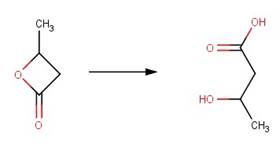
· Coumarin (El-Khatib and Nassr, 2007)
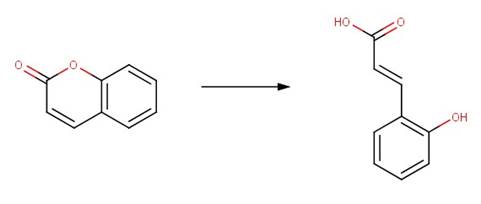
REFERENCES:
Bowden, K.; R.J. Ranson, A. Perjessy, M. Lacova, O. Hritzova, and W.W.F. Fabian. 1998. Base-catalyzed hydrolysis of gamma-lactones: reactivity-structure correlations for 3-(substituted phenoxy and thiophenoxymethylene)-(Z)-1(3H)-isobenzofuranones. J. Phys. Org. Chem. 11: 467-474.
El-Khatib, R.M. and L.A.M.E. Nassr. 2007. Reactivity trends of the base hydrolysis of coumarin and thiocoumarin in binary aqueous-methanol mixtures at different temperatures. Spectrochimica Acta Part A. 67: 643-648.
Olson, A.R. and P.V. Volue. 1951. The Hydrolysis of Beta-Butyrolactone. J. Am. Chem. Soc. 73: 2468-2471.
Pocker, Y. and E. Green. 1973. Hydrolysis of D-glucono-delta-lactone. I. General Acid-Base Catalysis, Solvent Deuterium Isotope Effects, and Transition State Characterization. J. Am. Chem. Soc. 95: 113-119.
Weeks, D.P. and D.B. Whitney. 1981. Hydrolysis of 3-(m-nitrophenyl)-3-methoxyphthalide. J. Am. Chem. Soc. 103: 3555-3558.
SCHEME:
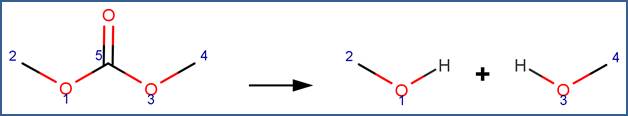
Note that a selectivity rule is included for this scheme to eliminate duplication of products. Specifically, to distinguish between the carbon atoms labelled 1 and 3, atom 1 is identified as the less sterically hindered atom. Additionally, to distinguish this scheme from the Cyclic Carbonate scheme, the scheme includes a reactivity rule which specifies that atom 5 is a chain atom.
EXAMPLES:
· Spirotetramat (EFSA, 2008)
REFERENCES:
EFSA (European Food Safety Authority). 2008. Draft Assessment Report (DAR): Joint Review Project/ OECD Monograph on Spirotetramat provided by the regulatory authorities of Austria, Canada and the United States of America, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
SCHEME:
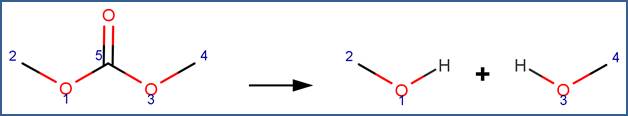
This scheme is similar to the Carbonate scheme, except that the scheme includes a reactivity rule which specifies that atom 5 is a ring atom.
EXAMPLES:
No examples were found for the Cyclic Carbonate Hydrolysis pathway.
REFERENCES:
N/A
SCHEME:
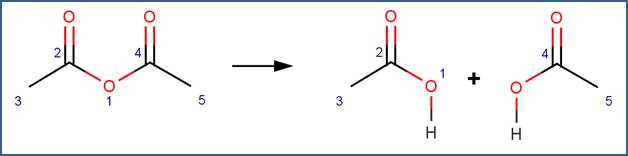
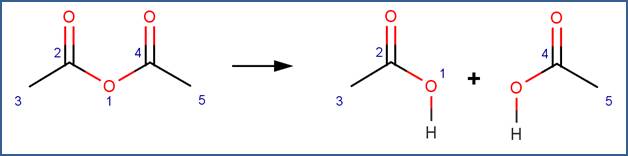
Note that a selectivity rule is included for this scheme to eliminate duplication of products. Specifically, to distinguish between the carbon atoms labelled 2 and 4, atom 2 is identified as the less sterically hindered atom.
EXAMPLES:
· Acetic anhydride (Bunton and Fendler, 1965)
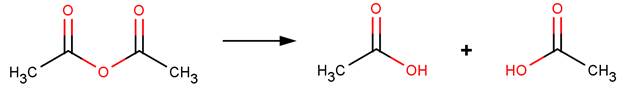
· Trimethylacetic anhydride (Bunton and Fendler, 1965)
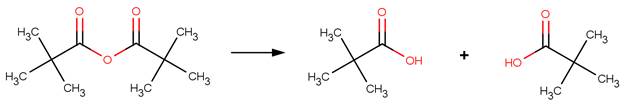
REFERENCES:
Bunton, C.A. and J.H. Fendler. 1965. The hydrolysis of carboxylic anhydrides. V. The acid hydrolysis of acetic and trimethylacetic anhydride. Journal of Organic Chemistry. 30(5): 1365-1371.
SCHEME:
This scheme is similar to the Anhydride scheme, except that the scheme includes a reactivity rule which specifies that atom 1 is a ring atom.
EXAMPLES:
· Glutaric anhydride (Bunton et al, 1963)
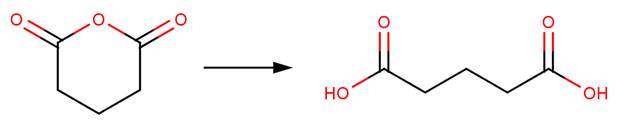
· Maleic anhydride (Bunton et al, 1963)
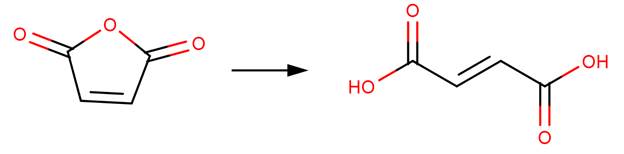
· Succinic anhydride (Bunton et al, 1963)
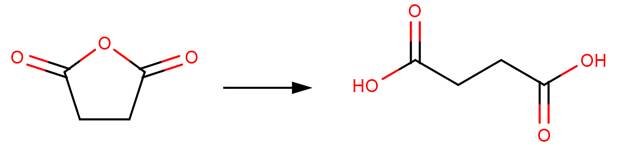
· Tetramethylsuccinic anhydride (Bunton et al, 1963)
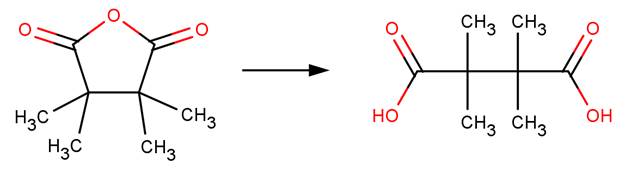
· 3,6-Dimethylphthalic anhydride (Hawkins, 1975a,b)
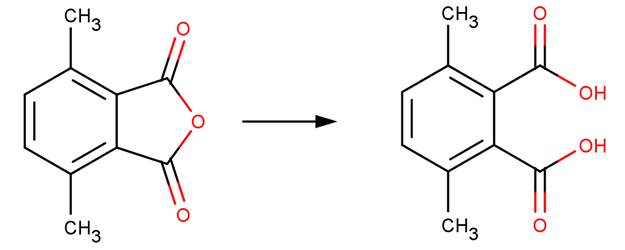
· Naphtho(2,3-c)furan-1,3-dione (Barros et al, 2001)
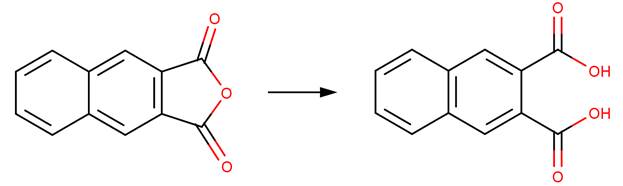
REFERENCES:
Barros, T.C., S. Yunes, G. Menegon, F. Nome, H. Chaimovich, M.J. Politi, L.G. Dias and I.M. Cuccovia. 2001. Hydrolysis of 1,8- and 2,3-naphthalic anhydrides and the mechanism of cyclization of 1,8-naphthalic acid in aqueous solutions. Journal of the Chemical Society, Perkin Transactions 2. 2001(12): 2342-2350.
Bunton, C.A., N.A. Fuller, S.G. Perry and V.J. Shiner. 1963. The hydrolysis of carboxylic anhydrides. Part III. Reactions in initially neutral solution. Journal of the Chemical Society. 1963: 2918-2926.
Hawkins M.D. 1975a. Hydrolysis of phthalic and 3,6-dimethylphthalic anhydrides. Journal of the Chemical Society, Perkin Transactions 2. 1975(4): 282-284.
Hawkins M.D. 1975b. Hydrolysis of 2,2,2-trifluoroethyl hydrogen 3,6-dimethylphthalate. Journal of the Chemical Society, Perkin Transactions 2. 1975(4): 285-287.
SCHEME:
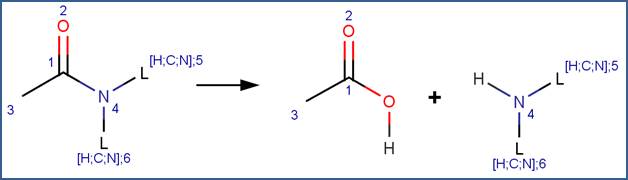
EXAMPLES:
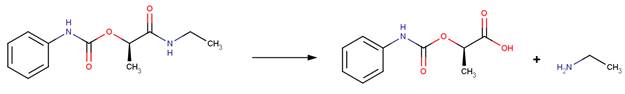
· Carbetamide (EFSA, 2006a)
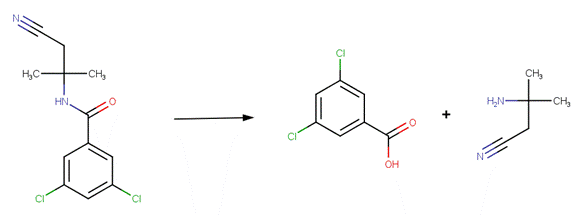
· Pronamide (Larson and Weber, 1994)
· Propanil (EFSA, 2008b)
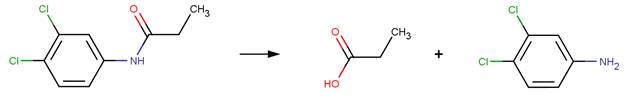
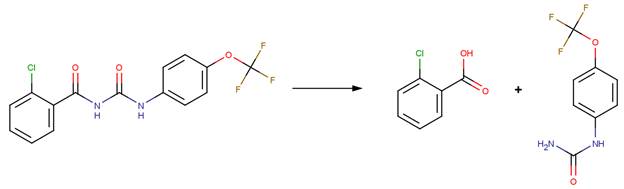
FORMYLUREA EXAMPLES: (Urea group adjacent to an amide group)
· Diflubenzuron (EFSA, 2006b)
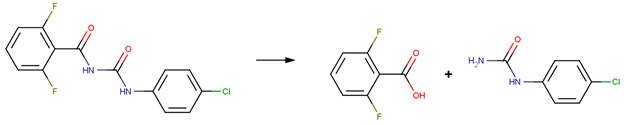
· Flufenoxuron (EFSA, 2008a)
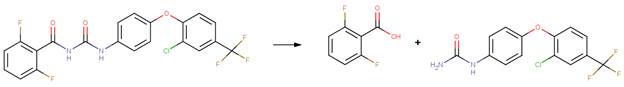
· Lufenuron (EFSA, 2007b)
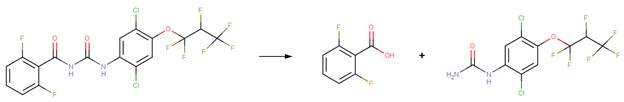
· Novaluron (EFSA, 2008c)
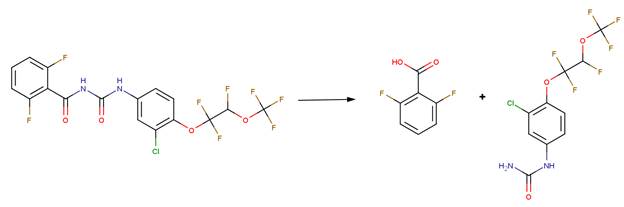
· Teflubenzuron (EFSA, 2007c)
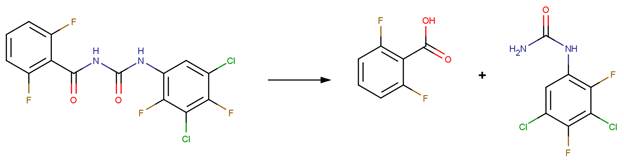
· Triflumuron (EFSA, 2007a)
REFERENCES:
EFSA (European Food Safety Authority), 2006a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State France for the existing active substance Carbetamide of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2006b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Sweden for the existing active substance Diflubenzuron of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.2 and B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2007a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Italy for the existing active substance Triflumuron of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2007b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Portugal for the existing active substance Lufenuron of the third stage (part B) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2007c. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the United Kingdom for the existing active substance Teflubenzuron of the third stage (part B) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2008a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State France for the existing active substance Flufenoxuron of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.2 and B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2008b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Italy for the existing active substance Propanil of the review programme referred to in Article 8(1) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2008c. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the United Kingdom for the existing active substance Novaluron of the review programme referred to in Article 8(1) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
Larson, R.A. and E.J. Weber. Reaction Mechanisms in Environmental Organic Chemistry. Boca Raton: CRC Press, Inc., 1994.
SCHEME:
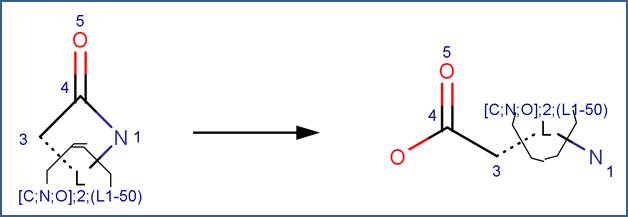
To distinguish this scheme from the Amide scheme, the scheme includes a reactivity rule which specifies that atom 1 is a ring atom. Additionally, to distinguish this scheme from hydrolysis of cyclic imides, the scheme includes a reactivity rule which specifies that atom 1 is not part of an imide structural fragment.
Studies comparing the rates of hydrolysis of lactam rings of various sizes indicate that the four-member β-lactam ring is more susceptible to hydrolysis than larger ring sizes (Bowden and Bromley, 1990; Imming et al, 2000; Wan et al, 1980); however, this trend is not currently captured in the hydrolysis reaction library. Lactam rings of four to 50 atoms are assigned the same rank.
EXAMPLES:
· 1-(4-Nitrophenyl)-2-azetidinone (Blackburn and Plackett, 1972)
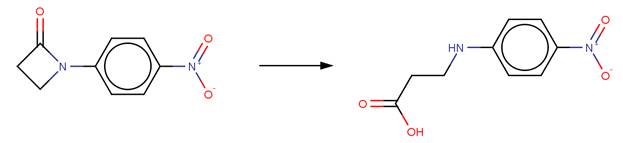
· 1-(3-Nitrophenyl)-2-pyrrolidinone (Bowden and Bromley, 1990)
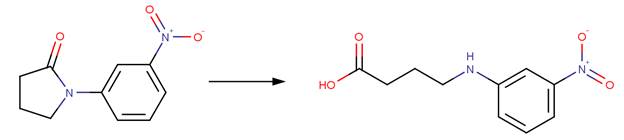
· Strychnine (Abbas et al, 1996)
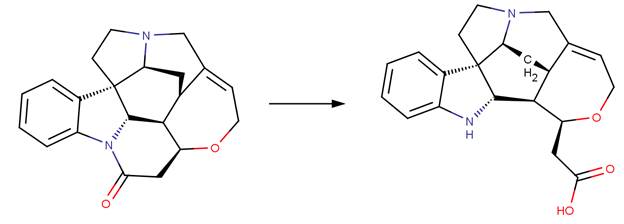
REFERENCES:
Abbas, K.A., P.Hurst and J.T. Edward. 1996. Reexamination of the Kirkwood-Westheimer theory of electrostatic effects. V. Effect of charged substituents on the rates of alkaline hydrolysis of substituted strychnines. Canadian Journal of Chemistry. 75: 441-448.
Blackburn, G.M. and J.D. Plackett. 1972. Strain effects in acyl transfer reactions. Part 1. The kinetics of hydrolysis of some N-aryl-lactams. Journal of the Chemical Society, Perkin Transactions 2. 1972(10): 1366-1371.
Bowden, K. and K. Bromley. 1990. Reactions of carbonyl compounds in basic solutions. Part 14. The alkaline hydrolysis of substituted N-Methylformanilides, N-Methylacetanilides, 1-Phenylazetidin-2-ones, 1-Phenyl-2-pyrrolidones, and 1-Phenyl-2-piperidones. Journal of the Chemical Society, Perkin Transactions 2. 1990(12): 2103-2109.
Imming, P., B. Klar and D. Dix. 2000. Hydrolytic stability versus ring size in lactams: Implications for the development of lactam antibiotics and other serine protease inhibitors. Journal of Medicinal Chemistry. 43(22): 4328-4331.
Wan, P., T.A. Modro and K. Yates. 1980. The kinetics and mechanism of acid catalysed hydrolysis of lactams. Canadian Journal of Chemistry. 58: 2423-2432.
SCHEME:
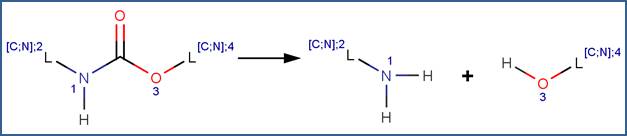
As is shown in the examples below, a number of N-alkyl and N-aryl carbamates have been observed to undergo hydrolysis according to the scheme shown above. The N,N-disubstituted carbamates (a.k.a. secondary carbamates) are resistant to hydrolysis (Aly and El-Dib, 1971; Christenson, 1964; EFSA, 2004c; Larson and Weber, 1994; Wolfe et al. 1978a).
EXAMPLES:
· 4-nitrophenyl N-methylcarbamate (Bender and Homer, 1965)
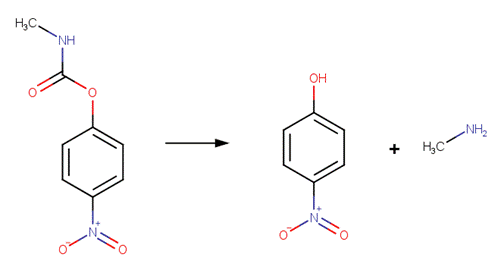
· Carbaryl (a.k.a. Sevin) (Aly and El-Dib, 1971; EFSA, 2005a; Wolfe et al, 1978b)
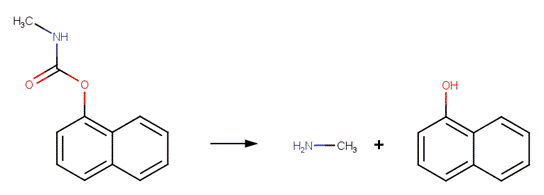
· Propoxur (a.k.a. Baygon) (Aly and El-Dib, 1971)
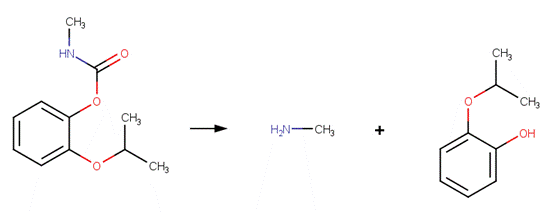
· Carbofuran (EFSA, 2004a; Iesce et al, 2006)
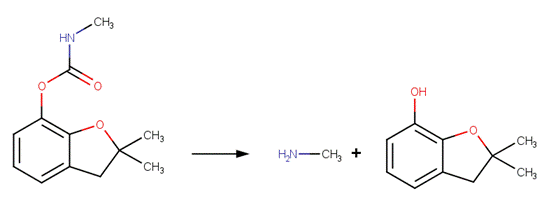
· Methiocarb (EFSA, 2005b)
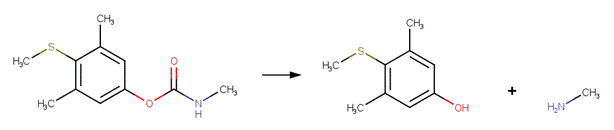
· Methomyl (EFSA, 2004b)
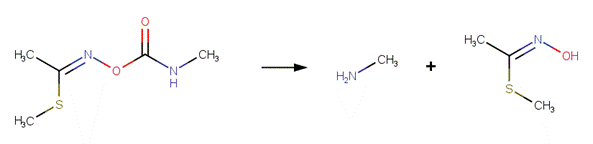
· Carbendazim (EFSA, 2009)
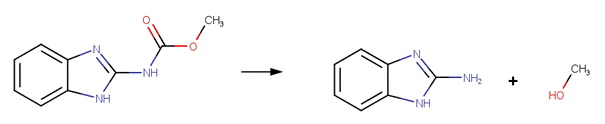
·
Carbetamide (EFSA, 2006)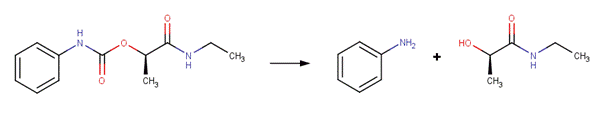
REFERENCES:
Aly, O.M. and M.A. El-Dib. 1971. Studies on the Persistence of Some Carbamate Insecticides in the Aquatic Environment. Water Research. 5: 1191-1205.
Bender, M.L. and R.B. Homer. 1965. The mechanism of the alkaline hydrolysis of p-nitrophenyl N-methylcarbamate. Journal of Organic Chemistry. 30(11): 3975-3978.
Christenson, I. 1964. Alkaline hydrolysis of some carbamic acid esters. Acta Chemica Scandinavica. 18(4): 904-922.
EFSA (European Food Safety Authority), 2004a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Belgium for the existing active substance Carbofuran of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part7, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2004b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State United Kingdom for the existing active substance Methomyl of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.6. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2004c. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State United Kingdom for the existing active substance Pirimicarb of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2005a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Spain for the existing active substance Carbaryl of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2005b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State United Kingdom for the existing active substance Methiocarb of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2006. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State France for the existing active substance Carbetamide of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority), 2009. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Germany for the existing active substance Carbendazim of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
Iesce, M.R., M. della Greca, F. Cermola, M. Rubino, M. Isidori and L. Pascarella. 2006. Transformation and Ecotoxicity of Carbamic Pesticides in Water. Environmental Science & Pollution Research 13(2): 105-109.
Larson, R.A. and E.J. Weber. Reaction Mechanisms in Environmental Organic Chemistry. Boca Raton: CRC Press, Inc., 1994.
Wolfe, N.L., R.G. Zepp, and D.F. Paris. 1978a. Use of Structure-Reactivity Relationships to Estimate Hydrolytic Persistence of Carbamate Pesticides. Water Research. 12: 561-563.
Wolfe, N.L., R.G. Zepp, and D.F. Paris. 1978b. Carbaryl, Propham and Chlorpropham: A Comparison of the Rates of Hydrolysis and Photolysis with the Rate of Biolysis. Water Research. 12: 565-571.
SCHEME:
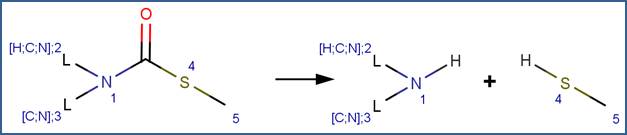
EXAMPLES:
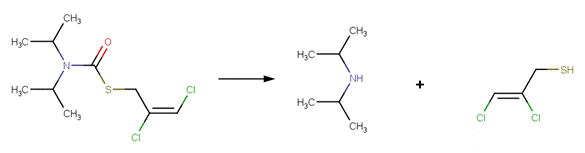
· Diallate (U.S. EPA, 1992, p. 88)
REFERENCES:
U.S. EPA (United States Environmental Protection Agency). 1992. Environmental fate constants for organic chemicals under consideration for EPA’s Hazardous Waste Identification Rule. EPA/601/R-92/006.
SCHEME:
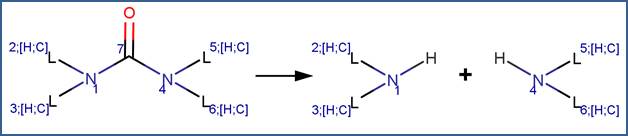
To distinguish this scheme from the Cyclic Urea Hydrolysis scheme, the scheme includes a reactivity rule which specifies that atom 7 is a chain atom. Additionally, a selectivity rule is included for this scheme to eliminate duplication of products. Specifically, to distinguish between the nitrogen atoms labelled 1 and 4, atom 1 is identified as the less sterically hindered atom.
In molecules with a urea group adjacent to an amide group, hydrolysis is generally observed to follow the amide pathway (formylurea examples). However, at high temperature (> 50°C), significant formation of the products of the urea hydrolysis pathway has been observed (EFSA, 2006a; EFSA, 2006b; EFSA, 2014).
EXAMPLES:
· Hexythiazox (EFSA, 2006a)
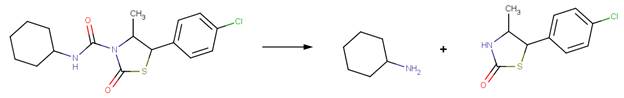
· Isoproturon (EFSA, 2014; Penning et al, 2008)
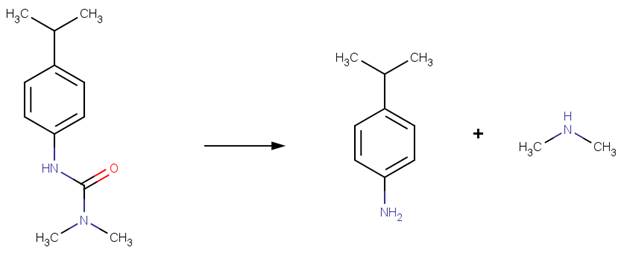
· Pencycuron (EFSA, 2006b)
REFERENCES:
EFSA (European Food Safety Authority). 2006a. Draft Assessment Report (DAR): Initial risk assessment provided by the rapporteur Member State Finland for the existing active substance Hexythiazox of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2006b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the Netherlands for the existing active substance Pencycuron of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2014. Renewal Assessment Report: Isoproturon, Volume 3, Annex B.8, Environmental fate and behavior. RMS: Germany, Co-RMS: Czech Republic. Available from http://dar.efsa.europa.eu/dar-web/provision.
Penning, H., C.J. Cramer and M. Elsner. 2008. Rate-dependent carbon and nitrogen kinetic isotope fractionation in hydrolysis of isoproturon. Environmental Science and Technology. 42(21): 7764-7771.
SCHEME:
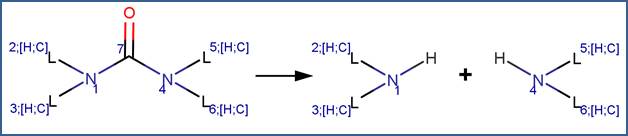
This scheme is similar to the Urea scheme, except that the scheme includes a reactivity rule which specifies that atom 7 is a ring atom.
EXAMPLES:
No examples were found for the Cyclic Urea Hydrolysis pathway.
REFERENCES:
N/A
SCHEME:
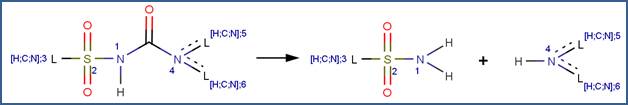
EXAMPLES:
· Metsulfuron-methyl (EFSA, 2013)
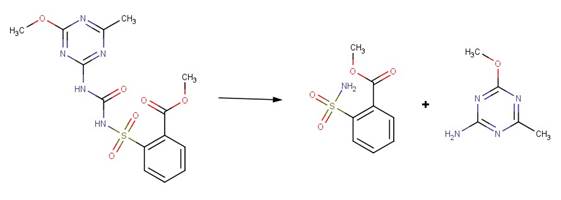
· Halosulfuron-methyl (EFSA, 2011)
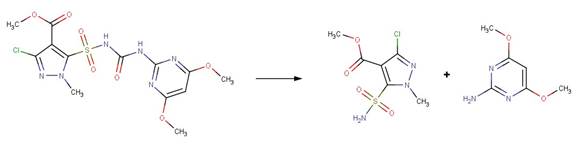
· Triflusulfuron-Methyl (EFSA, 2007a)
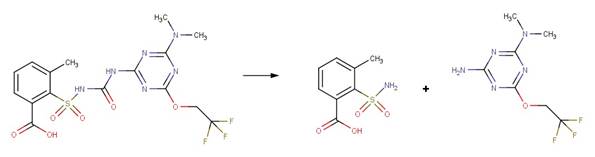
· Rimsulfuron (EFSA, 2005)
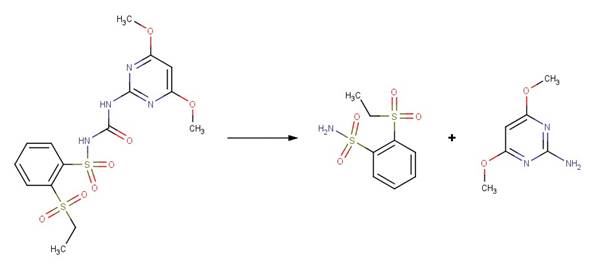
· Chlorsulfuron (EFSA, 2007b)
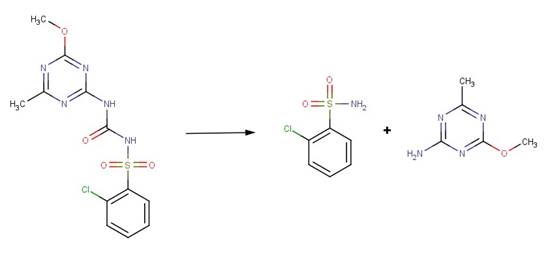
· Nicosulfuron (EFSA, 2006c)
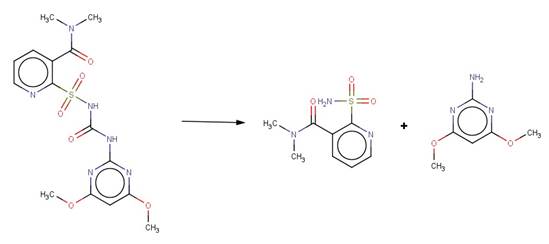
· Thiencarbazone-Methyl (EFSA, 2012).
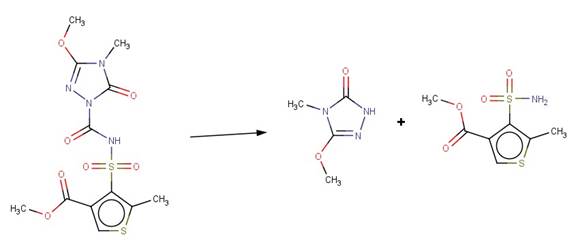
· Azimsulfuron (Boschin et al, 2007; EFSA, 2009)
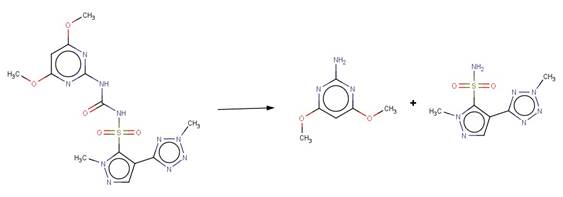
· Bensulfuron Methyl (EFSA, 2006b)
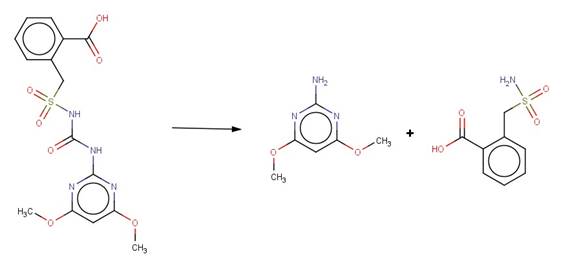
· Amidosulfuran (EFSA, 2006a)
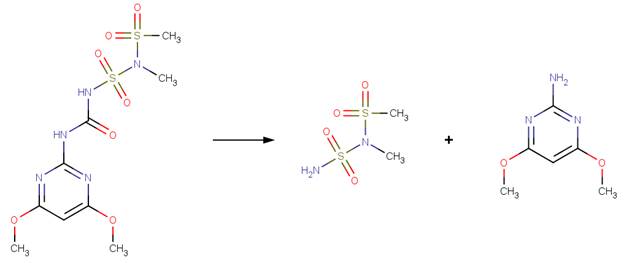
· Tribenuron-methyl (EFSA, 2004)
REFERENCES:
Boschin, G., A. D’Agostina, C. Antonioni, D. Locati and A. Arnoldi. 2007. Hydrolytic degradation of azimsulfuron, a sulfonylurea herbicide. Chemosphere. 68: 1312-1317.
EFSA (European Food Safety Authority). 2004. Draft Assessment Report (DAR): Initial risk assessment provided by the rapporteur Member State Sweden for the existing active substance Tribenuron (based on the variant tribenuron-methyl) of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2005. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Germany for the existing active substance Rimsulfuron of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2006a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Austria for the existing active substance Amidosulfuran of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2006b. Monograph prepared by the Member State Italy in the context of the inclusion of the following active substance Bensulfuron Methyl in Annex I of Council Directive 91/414/EEC, Volume 3, Annex B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2006c. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State The United Kingdom for the existing active substance Nicosulfuron of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2007a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State France for the existing active substance Triflusulfuron-Methyl of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2007b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Greece for the existing active substance Chlorsulfuron of the third stage (part B) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, part 4, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2009. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Sweden and the co-rapporteur Member State Slovenia for the existing active substance Azimsulfuron upon submission in the framework of renewal of the inclusion of a first group of active substances in Annex I to Council Directive 91/414/EEC, Volume 3, Annex B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2011. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Italy for the new active substance Halosulfuron-Methyl of the review programme referred to in Article 11(1) of Commission Regulation (EC) No 1107/2009, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2012. Report and Proposed Decision of the United Kingdom made to the European Commission for Thiencarbazone-Methyl under Regulation 1107/2009 (Article 80 transitional measures), Volume 3, Annex B, B.8, part A. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2013. Renewal Assessment Report (RAR): Draft Re-Assessment Report Review provided by the Member State Slovenia and the co-rapporteur Member State Sweden for the existing active substance Metsulfuron-methyl of Annex I inclusion under Regulation (EC) 1107/2009, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
SCHEME:
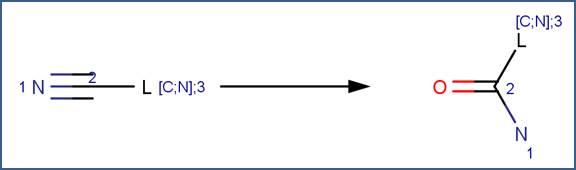
EXAMPLES:
· Acetonitrile (Peskoff and Meyer, 1913; U.S. EPA, 1987)

· Benzonitrile (Wiberg, 1955)
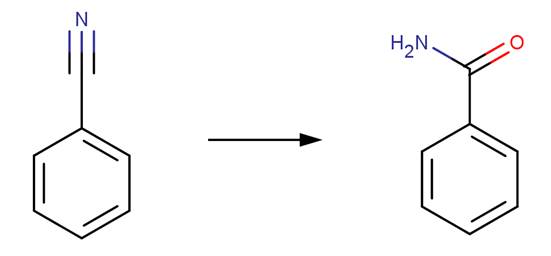
· 1,10‐phenanthroline‐2‐carbonitrile (Breslow et al, 1967)
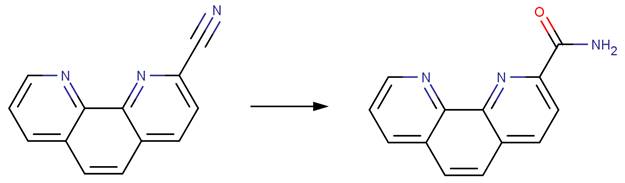
· Trichloroacetonitrile (Glezer et al, 1999)
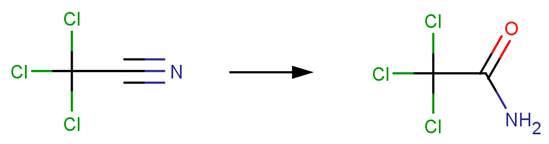
· Dichloroacetonitrile (Reckow et al, 2001)
· Dicyanamide (Hill et al, 1984)
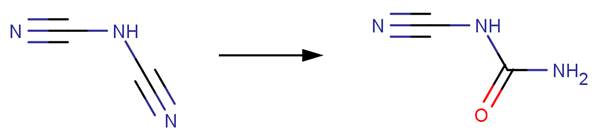
· Fipronil (EFSA, 2005a)
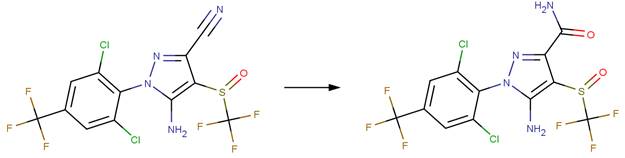
· Flonicamid (EFSA, 2005b)
REFERENCES:
Breslow, R., R. Fairweather and J. Keana. 1967. Metal-catalyzed hydration of phenanthroline nitrile. Journal of the American Chemical Society. 89: 2135-2138.
EFSA (European Food Safety Authority). 2005a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State France for the existing active substance Fipronil of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2005b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State France for the existing active substance Flonicamid of the third stage (part A) of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
Glezer, V., B. Harris, N. Tal, B. Iosefzon and O. Lev. 1999. Hydrolysis of haloacetonitriles: Linear free energy relationship, kinetics and products. Water Research. 33(8): 1938-1948.
Hill, S.V., A. Williams and J.L. Longridge. 1984. Acid-catalyzed hydrolysis of cyanamides: estimates of carbodi-imide basicity and tautomeric equilibrium constant between carbodi-imide and cyanamide. Journal of the Chemical Society, Perkin Transactions 2. 1984(6): 1009-1013.
Peskoff, N. and J. Meyer. 1913. Zur kenntnis der folgereaktion. III. Die hydrolyse von saureamiden und nitrile. Zeitschrift für Physikalische Chemie. 82: 129.
Reckow, D.A., T.L. Platt, A.L. MacNeill and J.N. McClellan. 2001. Formation and degradation of DCAN in drinking waters. AQUA. 50(1): 1-13.
U.S. EPA (United States Environmental Protection Agency). 1987. Measurement of hydrolysis rate constants for evaluation of hazardous waste land disposal: Volume 2. Data on 54 chemicals. EPA/600/3-87/019.
Wiberg, K.B. 1955. The mechanisms of hydrogen peroxide reactions. II. A comparison of the reactivity of hydroxyl ion and hydroperoxide ion toward benzonitrile. Journal of the American Chemical Society. 77: 2519-2522.
SCHEME:
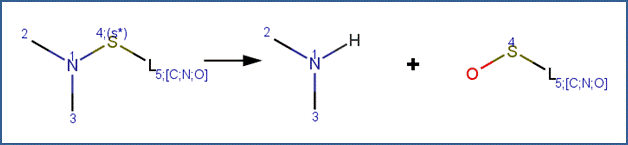
EXAMPLES:
· Benfuracarb (EFSA, 2004a; Iesce et al, 2006)
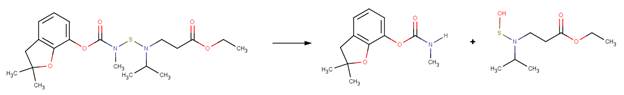
· Captan (EFSA, 2005a; Wolfe et al, 1976)
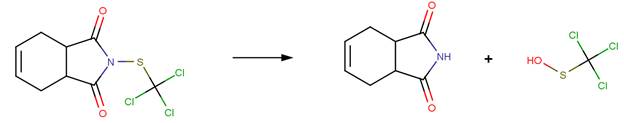
· Carbosulfan (de Melo Plese et al, 2005; EFSA, 2004b; Iesce et al, 2006; Umetsu et al, 1980)
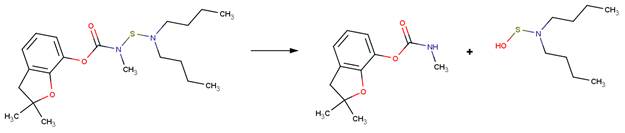
· Folpet (EFSA, 2005b)
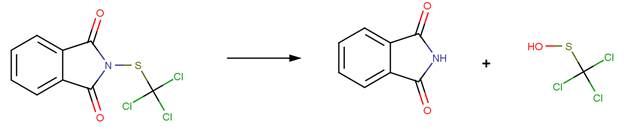
· Thiodicarb (EFSA, 2005c) The only major reported hydrolysis product of thiodicarb is methomyl (shown below). This product forms after two sequential N-S cleavage reactions. The first N-S cleavage results in a hydroxysulfanyl intermediate, which undergoes a second N-S cleavage reaction to form methomyl. The hydroxysulfanyl intermediate was not reported as an observed product in hydrolysis studies.
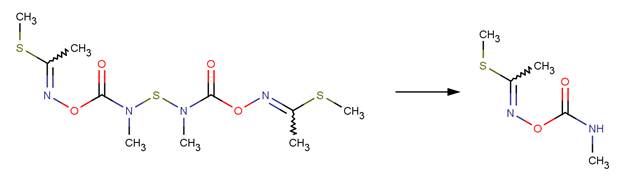
REFERENCES:
De Melo Plese, L.P., L.C. Paraiba, L.L. Foloni and L.R. Pimentel Trevizan. 2005. Kinetics of carbosulfan hydrolysis to carbofuran and the subsequent degradation of this last compound in irrigated rice fields. Chemosphere. 60: 149-156.
EFSA (European Food Safety Authority). 2004a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Belgium for the existing active substance Benfuracarb of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2004b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Belgium for the existing active substance Carbosulfan of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2005a. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Italy for the existing active substance Captan of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2005b. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State Italy for the existing active substance Folpet of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
EFSA (European Food Safety Authority). 2005c. Draft Assessment Report (DAR): Initial risk assessment provided by the Member State the United Kingdom for the existing active substance Thiodicarb of the second stage of the review programme referred to in Article 8(2) of Council Directive 91/414/EEC, Volume 3, Annex B, B.8. Available from http://dar.efsa.europa.eu/dar-web/provision.
Iesce, M.R., M. della Greca, F. Cermola, M. Rubino, M. Isidori and L. Pascarella. 2006. Transformation and Ecotoxicity of Carbamic Pesticides in Water. Environmental Science and Pollution Research. 13(2): 105-109.
Umetsu, N., E. Kuwano and T.R. Fukuto. 1980. Nature of N-S bond cleavage of 2,3-dihydro-2,2-dimethyl-7-benzofuranyl (di-n-butylaminosulfenyl) (methyl)carbamate. Journal of Environmental Science and Health B. 15(1): 1-23.
Wolfe, N.L., R.G. Zepp, J.C. Doster and R.G. Hollis. 1976. Captan hydrolysis. Journal of Agricultural and Food Chemistry. 24(5): 1041-1045.
SCHEME:
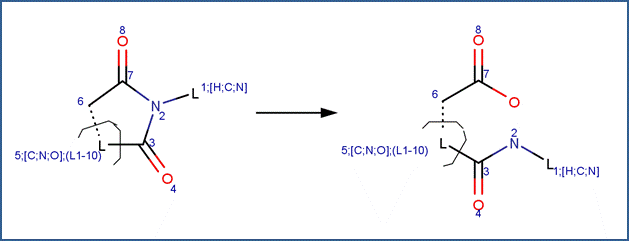
EXAMPLES:
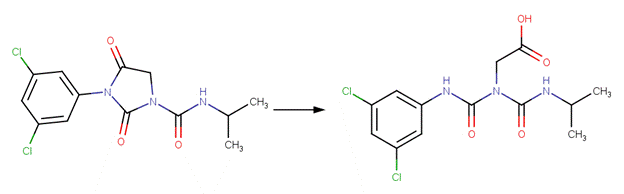
· Iprodione (Belafdal et al, 1986)
· N-phenylnaphthalimide (Donskikh et al, 1987)
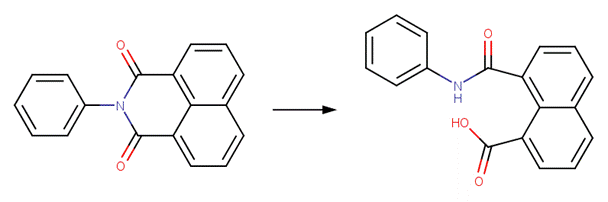
· N-(o-carboxyphenyl)-phthalimide (Donskikh et al, 1987)
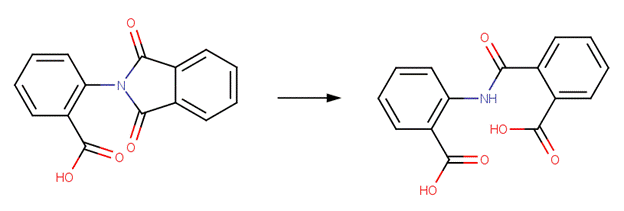
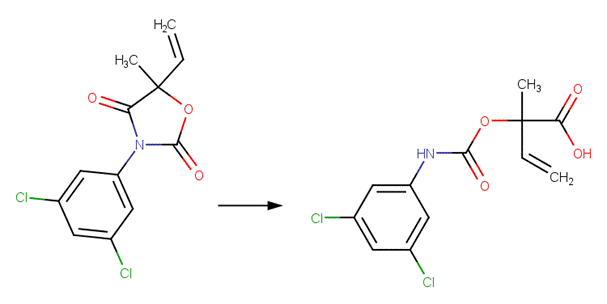
· Vinclozolin (Mercadier et al, 1998; Szeto et al, 1989)
REFERENCES:
Belafdal, O., M. Bergon and J.P. Calmon. 1986. Mechanism of hydantoin ring opening in iprodione in aqueous media. Pesticide Science. 17: 335-342.
Donskikh, A.I., O.I. Tomina, G.M. Tseitlin, Z.F. Saikina and J.E. Doroshenko. 1987. Hydrolytic stability of imides of different structures. Periodica Polytechnica Chemical Engineering. 33(1): 61-67.
Katritzky, A.R., J. Yao, M. Qi, Y. Chou, D.J. Sikora and S. Davis. 1998. Ring opening reactions of succinimides. Heterocycles. 48(12): 2677-2691.
Mercadier, C., D. Vega and J. Bastide. 1998. Chemical and biological transformation of the fungicide vinclozolin. Journal of Agricultural and Food Chemistry. 46(9): 3817-3822.
Szeto, S.Y., N.E. Burlinson, J.E. Rahe and P.C. Oloffs. 1989. Kinetics of the dicarboximide fungicide vinclozolin. Journal of Agricultural and Food Chemistry. 37(2): 523-529.
SCHEME:
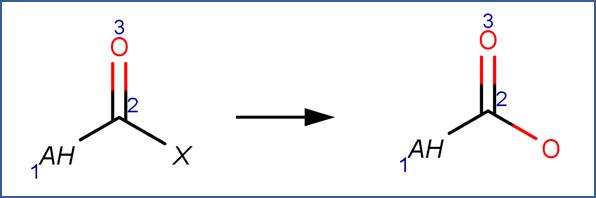
Hydrolysis of an acid halide yields a carboxylic acid through nucleophilic acyl substitution (McMurry, 2011, p. 830).
EXAMPLES:
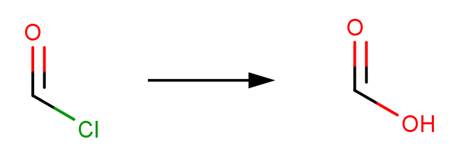
· Formyl chloride (Dowideit et al (1996), Prager et al, 2001)
· Acetyl chloride (Prager et al, 2001)

· Isopropyl chloroformate (Queen, 1967)

· S-Methyl chloridothiocarbonate (Queen et al, 1970)
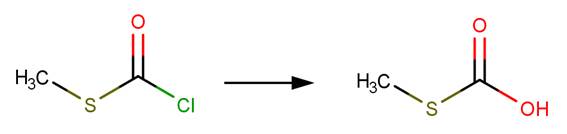
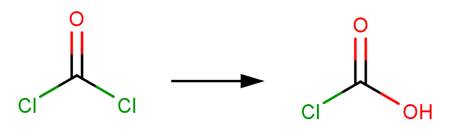
· Phosgene (Prager et al, 2001)
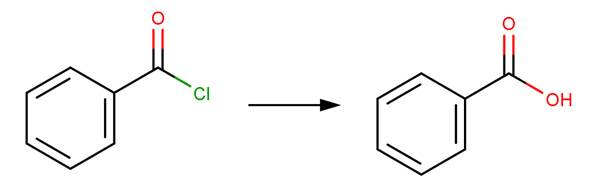
· Benzoyl chloride (Song and Jencks, 1989)
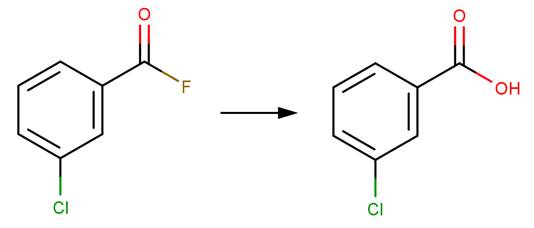
· 3‐Chlorobenzoyl fluoride (Song and Jencks, 1989)
REFERENCES:
Dowideit, P., R. Mertens and C. von Sonntag. 1996. Non-hydrolytic decay of formyl chloride into CO and HCl in aqueous solution. Journal of the American Chemical Society. 118: 11288-11292.
McMurry, J.E. 2011. Organic Chemistry, 8th ed. Boston, MA: Cengage Learning.
Prager, L., P. Dowideit, H. Langguth, H.-P. Schuchmann and C. von Sonntag. 2001. Hydrolytic removal of the chlorinated products from the oxidative free-radical-induced degradation of chloroethylenes: Acid chlorides and chlorinated acetic acids. Journal of the Chemical Society, Perkin Transactions 2. 2001: 1641-1647.
Queen, A. 1967. Kinetics of the hydrolysis of acyl chlorides in pure water. Canadian Journal of Chemistry. 45: 1619-1629.
Queen, A., T.A. Nour, M.N. Paddon-Row. 1970. Kinetics of the hydrolysis of thiochloroformate esters in pure water. Canadian Journal of Chemistry. 48: 522-527.
Song, B.D. and W.P. Jencks. 1989. Mechanism of solvolysis of substituted benzoyl halides. Journal of the American Chemical Society. 111: 8470-8479.
Ranking of Hydrolysis Reaction Schemes
The schemes within the hydrolysis library are ranked on a scale of one to seven according to their relative rate of transformation, with a higher rank indicating a faster hydrolysis rate. The pH-specific ranks in the table below were assigned based on the median half-life for all molecules included in a database of literature-reported rate data compiled for each scheme in the library.
|
Scheme |
pH 5 Rank |
pH 7 Rank |
pH 9 Rank |
|
Nucleophilic Substitution w/ no adjacent X |
3 |
3 |
3 |
|
Nucleophilic Substitution w/ vicinal X |
1 |
1 |
1 |
|
Nucleophilic Substitution w/ geminal X |
1 |
1 |
1 |
|
Elimination |
1 |
1 |
2 |
|
Epoxide Hydrolysis |
7 |
5 |
1 |
|
OP Ester Hydrolysis 1 (Base-Catalyzed) |
2 |
3 |
4 |
|
OP Ester Hydrolysis 2 (Neutral or Acid-Catalyzed) |
2 |
3 |
3 |
|
Carboxylic Acid Ester Hydrolysis |
2 |
2 |
5 |
|
Lactone Hydrolysis |
1 |
5 |
5 |
|
Carbonate Hydrolysis |
1 |
3 |
5 |
|
Cyclic Carbonate Hydrolysis |
1 |
3 |
5 |
|
Anhydride Hydrolysis |
7 |
7 |
7 |
|
Cyclic Anhydride Hydrolysis |
7 |
7 |
7 |
|
Amide Hydrolysis |
1 |
1 |
3 |
|
Lactam Hydrolysis |
4 |
4 |
4 |
|
Carbamate Hydrolysis |
3 |
3 |
5 |
|
Thiocarbamate Hydrolysis |
1 |
1 |
1 |
|
Urea Hydrolysis |
3 |
2 |
2 |
|
Cyclic Urea Hydrolysis |
3 |
2 |
2 |
|
Sulfonylurea Hydrolysis |
3 |
2 |
2 |
|
Nitrile Hydrolysis |
4 |
5 |
6 |
|
N-S Cleavage |
5 |
5 |
5 |
|
Imide Hydrolysis |
2 |
5 |
5 |
|
Acid Halide Hydrolysis |
7 |
7 |
7 |
The following table defines the seven ranking levels, which span residence times of environmental relevance.
|
Rank |
Range of Median Hydrolysis Half-Life |
|
7 |
Less than 30 minutes |
|
6 |
30 minutes to 200 minutes |
|
5 |
200 minutes to 24 hours |
|
4 |
24 hours to 7 days |
|
3 |
7 days to 60 days |
|
2 |
60 days to 1 year |
|
1 |
Greater than 1 year |
Version 1.7 of the CTS Hydrolysis Reaction Library was included in the initial public release of CTS (version 1.0). The development of the version 1.7 library was described in detail in the following publication:
C. Tebes-Stevens, J.M. Patel, W.J. Jones, E.J. Weber. 2017. Prediction of Hydrolysis Products of Organic Chemicals under Environmental pH Conditions. Environmental Science and Technology, 51(9), pp. 5008-5016.
Version 1.8 Revisions:
· The number of ranks was expanded from six to seven ranks to allow for greater resolution of the likelihood of production and accumulation of products formed by very fast reactions. The range of hydrolysis half-lives remained the same for ranks one through four; however, the range of half-lives associated with ranks five and six were modified slightly, and rank seven was introduced for schemes with median observed half-lives less than 30 minutes. As a result of the new rank definitions, rank assignments increased by one for Anhydride Hydrolysis, Cyclic Anhydride Hydrolysis, and Acid Halide Hydrolysis across all pH values. Rank assignments also increased by one for Epoxide Hydrolysis at pH 5 and Nitrile Hydrolysis at pH 9.
· The Halogenated Aliphatics: Elimination scheme was modified so that the reaction would not proceed for fluorinated aliphatics. Specifically, reactant atom 3 was changed from a halogen (X) to a list atom ([Cl;Br;I]). Under environmentally relevant conditions, the abiotic elimination reaction would not be expected to proceed for fluorinated aliphatics due to the strength of the carbon-fluorine bond.
· The Dehydration of Geminal Diols scheme was removed from the CTS Abiotic Hydrolysis Library and placed in the CTS Spontaneous Reaction Library, because it is not a true hydrolysis reaction. The Spontaneous Reaction Library contains aqueous reactions that occur very quickly and is intended to be combined with other CTS reaction libraries. Whenever transformations are predicted using the CTS Abiotic Hydrolysis Library in CTS, predictions from the CTS Spontaneous Reaction Library will be included in the results.